ACCURATE. COMPLIANT. ADAPTABLE.
Our Mission is Clear — expedite therapies by streamlining archaic processes, reducing costs, and accelerating complex treatments to patients with Luminari’s comprehensive toolbox that includes LumiPath™ AI software.
LumiPath™ doesn't just automate clinical protocol generation - it transforms it. Through specialized AI agents with deep regulatory and therapeutic expertise, we're delivering breakthrough improvements that compress development timelines, reduce costs, and accelerate patient access to life-changing therapies. The numbers tell the story of a fundamental shift in how drugs move from discovery to patients.
Reduce clinical trial timelines through AI optimization
Lower development costs via streamlined processes
Blockchain-secured immutable trial data
Accelerated recruitment
through AI matching
Revolutionary technology stack transforming pharmaceutical development through AI and digital twin technologies.

Our proprietary AI platform streamlines clinical trial processes, eliminates errors and redundancies, and ensures the integrity of study data and outcomes.
Our virtual patient simulation tool provides real-time insights into trial dynamics, empowering sponsors to make informed decisions and optimize study design.

By leveraging our custom-trained AI models, sponsors can quickly identify gaps, forecast desired outcomes, and make real-time adjustments - driving efficiency and ensuring trial success.
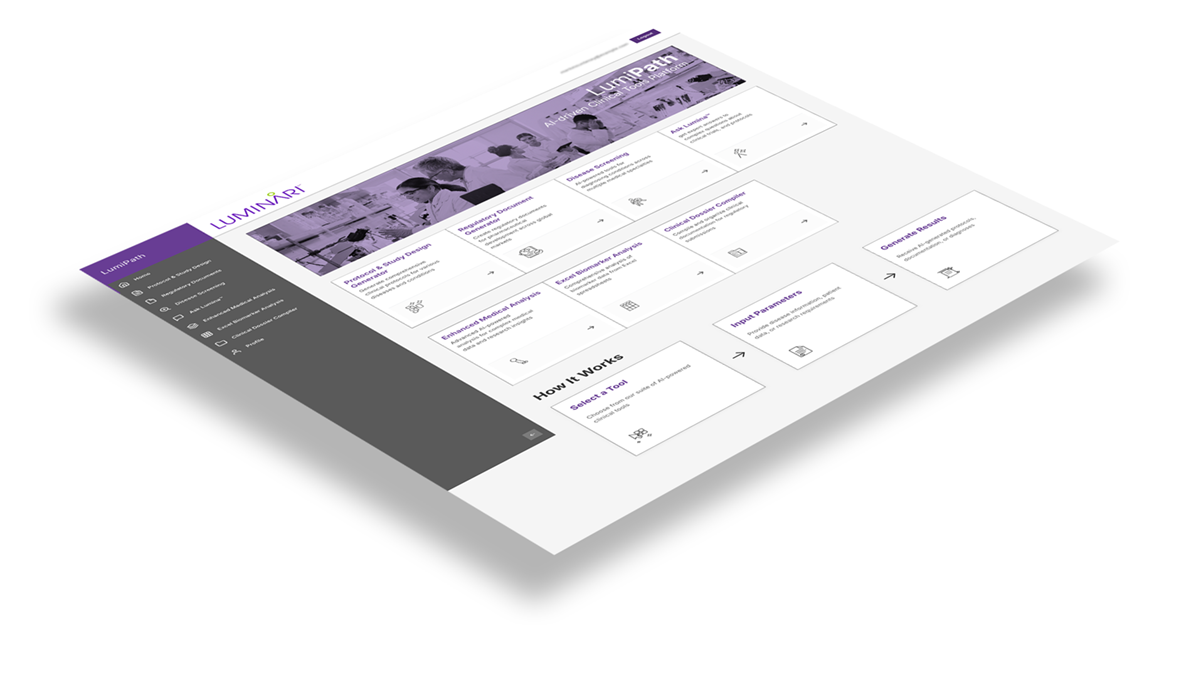

LumiPath™ eliminates the protocol development bottleneck that delays drug development and postpones patient access to critical therapies. Our multi-agent AI platform generates regulatory-compliant clinical trial protocols in minutes—complete with optimized statistical designs, comprehensive safety monitoring plans, and operational execution frameworks that reduce the 60% amendment rate plaguing today's trials.
Experience the power of AI agents trained on thousands of successful protocols across therapeutic areas, ensuring your submissions meet rigorous FDA, EMA, and ICH standards while incorporating best practices that accelerate approval timelines and trial success rates.
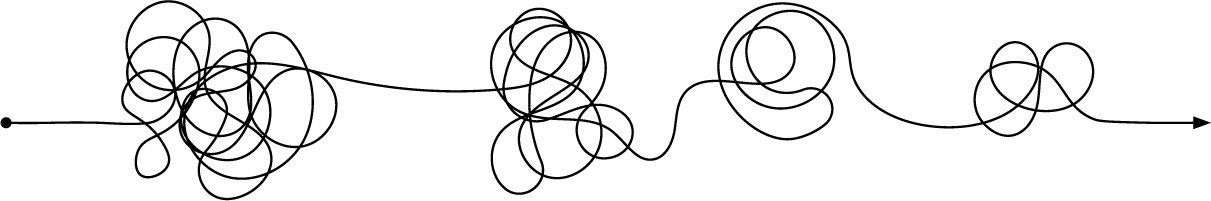
Revolutionary technology stack transforming pharmaceutical development through AI and digital twin technologies.







We invite you to challenge our system with your current protocol development needs. Provide the same inputs you would use in your traditional process and compare the results directly. Put Us To The Test.
By harnessing the power of artificial intelligence we are creating innovative solutions that drive unprecedented efficiency, accessibility, and patient-centricity throughout the drug development process.
Our team of industry experts and technology pioneers are dedicated to accelerating the path from discovery to delivery. We empower pharmaceutical companies, research organizations, and patients tocollaborate like never before, unlocking new possibilities in the pursuit oflife-changing therapies.
Our proprietary AI solutions are leading the transformation of the pharmaceutical industry. We envision a future where cutting-edge treatments are brought to market swiftly, with precision and affordability. Our virtual patient models predict study outcomes and optimize every step of the clinical trial journey.
Our vision is to be the catalyst for groundbreaking advancements in drug development.
Everyday clinical protocols remain in development is another day patients wait for life-changing therapies. We've built AI technology that transforms 8-week protocol generation into 8 minutes—without compromising quality.
If you're ready to accelerate development timelines, reduce costly amendments, and bring therapies to patients faster, let's talk about how LumiPath™ can transform your clinical development process!