ACCURATE. COMPLIANT. ADAPTABLE.
Our Mission is Clear — expedite therapies by streamlining archaic processes, reducing costs, and accelerating complex treatments to patients with Luminari’s comprehensive toolbox that includes LumiPath™ AI software.
LumiPath™ doesn't just automate clinical protocol generation - it transforms it. Through specialized AI agents with deep regulatory and therapeutic expertise, we're delivering breakthrough improvements that compress development timelines, reduce costs, and accelerate patient access to life-changing therapies. The numbers tell the story of a fundamental shift in how drugs move from discovery to patients.
Reduce clinical trial timelines through AI optimization
Lower development costs via streamlined processes
Blockchain-secured immutable trial data
Accelerated recruitment
through AI matching
Revolutionary technology stack transforming pharmaceutical development through AI and digital twin technologies.

Our proprietary AI platform streamlines clinical trial processes, eliminates errors and redundancies, and ensures the integrity of study data and outcomes.
Our virtual patient simulation tool provides real-time insights into trial dynamics, empowering sponsors to make informed decisions and optimize study design.

By leveraging our custom-trained AI models, sponsors can quickly identify gaps, forecast desired outcomes, and make real-time adjustments - driving efficiency and ensuring trial success.
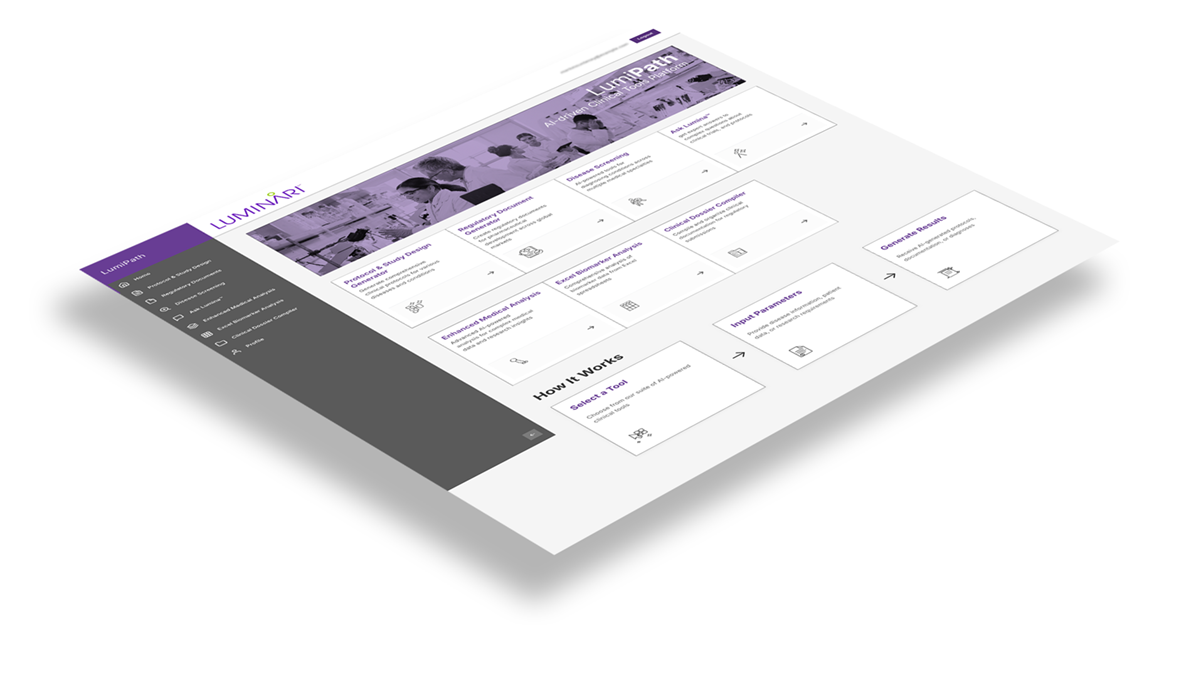

LumiPath™ eliminates the protocol development bottleneck that delays drug development and postpones patient access to critical therapies. Our multi-agent AI platform generates regulatory-compliant clinical trial protocols in minutes—complete with optimized statistical designs, comprehensive safety monitoring plans, and operational execution frameworks that reduce the 60% amendment rate plaguing today's trials.
Experience the power of AI agents trained on thousands of successful protocols across therapeutic areas, ensuring your submissions meet rigorous FDA, EMA, and ICH standards while incorporating best practices that accelerate approval timelines and trial success rates.
Revolutionary technology stack transforming pharmaceutical development through AI and digital twin technologies.







We invite you to challenge our system with your current protocol development needs. Provide the same inputs you would use in your traditional process and compare the results directly. Put Us To The Test.
By harnessing the power of artificial intelligence we are creating innovative solutions that drive unprecedented efficiency, accessibility, and patient-centricity throughout the drug development process.
Our team of industry experts and technology pioneers are dedicated to accelerating the path from discovery to delivery. We empower pharmaceutical companies, research organizations, and patients tocollaborate like never before, unlocking new possibilities in the pursuit oflife-changing therapies.
Our proprietary AI solutions are leading the transformation of the pharmaceutical industry. We envision a future where cutting-edge treatments are brought to market swiftly, with precision and affordability. Our virtual patient models predict study outcomes and optimize every step of the clinical trial journey.
Our vision is to be the catalyst for groundbreaking advancements in drug development.
Powerhouse Front Bench.

20+ years of experience in Healthcare and life sciences.
Launched more than 35 products globally, across a variety of TA’s, including but not limited to Oncology, Neurology, Rare Disease and others.
Worked with PwC, CVS Health, BioSolutia/Caremetx, Parexel, Amgen and others.
Numerous Technology Implementation/ Digital Transformation, Operational Efficiency, and Innovative Product Development and Utilization.

Strategic Advisor – client advocate and relationship builder
Life sciences executive with over 22 years expertise in data analytics, RWD/RWE, clinical research, market access, leadership and strategic partnerships.
Proven success driving business opportunities, managing key alliances, and exceeding sales and growth targets.
Core strengths in clinical research, commercialization, real-world data/evidence, AI, business development, strategic planning, and sales cycle and pipeline management.

Chief Data Officer – in-house geek and technology maven
Executive Advisor l Information & Decision Scientist l Operations Management
Worked on over 100 new projects with entrepreneurs and companies across the globe in numerous industries, including manufacturing, health and medical, telecommunications, information technology.
Academic rank of Professor in the Managerial Economics & Decision Sciences and Operations Management department.

Executive advisor and Chief Learning Officer
Executive Advisor SFU Venture Labs, startup coach, Chief Learning Officer (CLO) and former IBM Executive, Chief Operating Officer, Associate Faculty member, and TED Talker, constantly looking for ways to accelerate opportunity.
Chuck focuses on Learning Strategies, AI, Blockchain, Web3, and Digital Transformation.

More than 30 years of R&D leadership across the pharmaceutical and biotechnology industries.
Has previously served as Head of R&D IT/CIO for MedImmune, AstraZeneca’s biologics division, and later led Data Science and AI for AstraZeneca.
Has held senior roles at Schrödinger, Accelrys, GlaxoSmithKline, and Merck & Co. His career began with undergraduate research at Penn State, followed by roles at Tektagen (Charles River Laboratories), the Pennsylvania State Police as a forensic scientist, and STC (Orasure) as an R&D scientist. He holds degrees in biochemistry and molecular biology from Pennsylvania State University and Lehigh University.
Everyday clinical protocols remain in development is another day patients wait for life-changing therapies. We've built AI technology that transforms 8-week protocol generation into 8 minutes—without compromising quality.
If you're ready to accelerate development timelines, reduce costly amendments, and bring therapies to patients faster, let's talk about how LumiPath™ can transform your clinical development process!